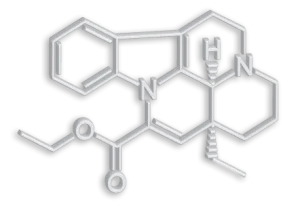
Vinpocetine is a semi-synthetic derivative of vincamine, an alkaloid derived from Vinca minor L. that has been used since the 1970s in Japan, Europe, Mexico and Russia for the treatment of cognitive and cerebrovascular disorders.
Vinpocetine may also be derived from tabersonine, an alkaloid extracted from the seeds of African Voacanga.
Covex S.A. is a global manufacturer of Vinpocetine, our facilities located in Europe (Spain), make our Vinpocetine meet the highest standards. Using a proprietary patent (ES 549.105) to obtain vinpocetine through tabersonine, an alkaloid extracted from the African Voacanga.
Vincamine (VC) is also an alkaloid of the eburnamenine type.
– CAS NO: 1617-90-9
– EC NO: 216-576-3
– Molecular formula: C21H26N2O3
– (CVX-DMF-100)
Identification and chemical analysis
– Generic Name (C.D.N.): Vinpocetine
– Chemical name: Eburnamenine (3α,16α)-14- carboxylic acid ethyl ester. Ethyl apovincaminate
– CAS RN: 42971-09-5
– Laboratory code: DMF-400
Similar standards for the identity and quality of vinpocetine products have been specified by the United States, Great Britain and European pharmacopoeias.
Covex Vinpocetine specifications
| C22H26N2O2 | |
| [42971-09-5] | NW |
| 350.45 | |
| DEFINITION | |
| Eburnamenine-14-carboxylic acid, ethyl ester, (3α,16α)-; Ethyl apovincamin-22-oate. | |
| ANALYSIS | SPECIFICATIONS |
| CHARACTERISTICS | |
| Appearance: | White or slightly yellow, crystalline powder |
| Solubility: | Practically insoluble in water, soluble in methylene chloride, slightly soluble in anhydrous ethanol. |
| IDENTIFICATION | |
| A. Specific optical rotation: | +127.0° to +134.0° (dry substance). |
| B. Infrared absorption spectrophotometry. | According to Vinpocetine CRS. |
| C. HPLC | The retention time of the main peak of the sample solution corresponds to that of the main peak for the Stock standard solution, as obtained in the organic impurities test. |
| RELATED COMPOUNDS, (HPLC) | A) Ethyl vincaminate: Not more than. B) Apovincamine: Not more than 0.5%. C) Methoxyvinpocetine: Not more than 0.3%. D) Dihydrovinpocetine: Not more than 0.5%. Unspecified impurities, each: Not more than 0.10%. Total impurities: Not more than 1.0%. |
| LOSS THROUGH DESICCATION | Not more than 0.5% of its weight. |
| SULPHATED ASH (Residue from ignition) | Not more than 0.1%. |
| HEAVY METALS | Not more than 10 ppm. |
| ACIDIMETRIC ASSAY (Titrimetry) | Not less than 98.5% and not more than 101.5% of C22H26N2O2, calculado sobre la sustancia seca. |
| RESIDUAL SOLVENTS | Etanol ≤ 2000 ppm. (0.20%). Methylene chloride ≤ 100 ppm. (0.01%). |
| CONTROL OF MICROBIOLOGICAL CONTAMINATION: | |
| TAMC | ≤ 103 CFU / 1 g. |
| TYMC | ≤ 102 CFU / 1 g. |
| Gram-bile tol. | ≤ 10 CFU / 1 g. |
| Escherichilla coli | Missing / 1 g. |
| St. Aureus | Missing / 1 g. |
| Ps. Aeruginosa | Missing / 1 g. |
| Salmonela | Missing / 10 g. |
| PYROGEN | Apyrogens |
Restricted Area - Authorized Personnel Only
By accessing this website you accept and agree to the Data Protection and Legal Information. In order to comply with Spanish legislation on Advertising of Medicinal Products for Human Use, we must inform you that the information below is intended for health professionals.
By accessing this area a cookie is installed in your computer (or device), for more information on cookies please visit our cookie policy.
If you are not a healthcare professional, please refrain from accessing this website.
